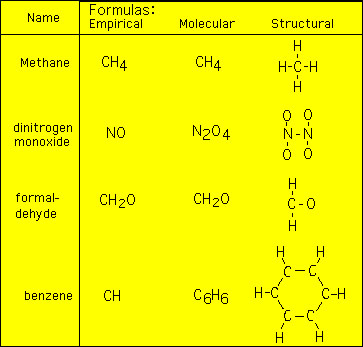
Empirical Formula of Benzene
The empirical formula is the simplest whole number ratio defining constituent atoms in a speciesand thus for benzene whose. N The molecular formula of benzene is C6H6.
What Is The Empirical Formula Of Benzene Quora
Molecular formula of benzene C 6 H 6 Molecular formula mass of benzene 78 Empirical formula of benzene C H Empirical formula mass of benzene 13 empirical formula.
. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom. The empirical formula of benzene is CH its molecular formula is C6H6. Empirical Formula of benzene C6H6- CH ascorbic acid C6H8O6- sucrose C12H22O11- C12H22O11 naphthalene C10H4- hydrochloric acid HCI- Which model do you prefer to.
Benzene is an organic chemical compound with the molecular formula C 6 H 6. Thus the empirical formula of Benzene is CH2. Empirical formula of benzene C6H6 is the molecular formula for benzene.
However the molecular formula is a depiction of the compounds actual whole number ratio. The empirical formula is the lowest possible whole number of the ratio between the atoms of elements in the compound. The atoms are.
See full answer below. Benzene is an organic compound. So we got acetylene H C C H.
Hence the empirical formula of benzene will. Now ratio of Carbon atoms to Hydrogen. The molecular formula of benzene is C6H6 the empirical formula of benzene is CH.
What is the empirical formula for C6H6. What is the empirical formula for benzene. The empirical formula of benzene is CH its molecular formula is C6H6.
The empirical formula of benzene is CH its chemical formula. The molecular formula of benzene is C6H6 the empirical formula of benzene is CH. The empirical formula of benzene eqC_6H_6 eq is CH.
After dividing the subscripts by 6 we will get 1 for each atom. Benzene Molecular formula C6H 6. Empirical formula of a chemical compound is a representation of the simplest whole number ratio between the elements comprising the compound.
Benzene C6H6 CID 241 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information. The GCF of the subscripts is 6. C 2 H 2 The empirical formula is the smallest whole number ratio of the number of atoms present in the element of the given compound.
C 6 H 6 Acetylene. As we known that empirical formula shows the simplest whole number ratio of the atoms. The molecular formula is C 2 H 2 empirical formula is C H.
And we got benzene C 6 H 6 the empirical formula is C H. The empirical formula of benzene is CH its molecular formula is C 6 H 6. In fact if you note the general formula of an alkene you can clearly see that the empirical formula for all alkenes will indeed.

The Empirical Formula Of Benzene And Acetylene Is Are Youtube
Quantitative Chemistry Molecular Formulas

C6h6 Lewis Structure Benzene Lewis Chemical Formula Home Decor Decals

0 Response to "Empirical Formula of Benzene"
Post a Comment